Organic Letters 誌に掲載
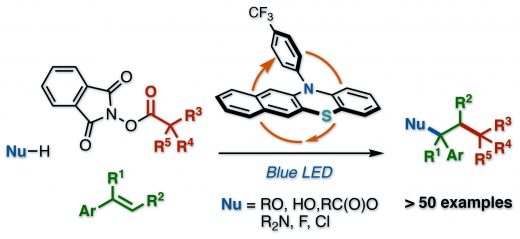
Our new organophotoredox-catalyzed radical relay for vicinal difunctionalization of alkenes using heteroatom nucleophiles and aliphatic redox active esters has been published to Org. Lett. This manuscript describes a visible-light-mediated organophotoredox catalytic process for vicinal difunctionalization of alkenes using heteroatom nucleophiles and aliphatic redox active esters. A wide range of heteroatom nucleophiles including alcohols, water, carboxylic acids, amides, and halogens can be used for this reaction. This radical relay type reaction allows forging of C(sp3)–C(sp3) with a carbon-centered radical and C(sp3)–heteroatom bonds with a benzyl cation on the vinylarenes with complete regioselectivity in a single step.
DOI: 10.1021/acs.orglett.1c00211