Catalysis
Radical Catalysis
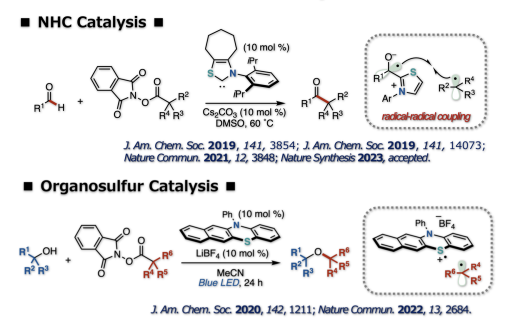
We developed new radical catalysis. This is based on the catalyst design focusing on the reaction pathway involving a single electron transfer and the subsequent radical-mediated bond formation. For example, N-heterocyclic carbene (NHC) catalysis promoted the decarboxylative coupling of aldehydes and tertiary or secondary alkyl carboxylic acid-derived redox-active esters to produce ketones. A reaction pathway involving single electron transfer from an enolate form of Breslow intermediate to a redox ester followed by recombination of the resultant radical pair to form a carbon–carbon bond is proposed. Visible light-mediated organosulfur catalysis enabled decarboxylative coupling between simple aliphatic alcohol, amine or thiol nucleophiles and tertiary or secondary alkyl carboxylic acid-derived redox active esters to produce a C(sp3)–X–C(sp3) fragment. The reaction involves a radical-polar crossover process, which enables the generation of a carbocation from a carbon radical.
The direct visible-light excitation of organoboronate complex generated alkyl radicals without the need for an external photoredox catalyst. The photoexcitation of the borates is applicable to cross-couplings, thus enabling the introduction of various C(sp3) fragments to organic molecules. A visible light-driven triple photoredox/cobalt/Brønsted acid catalysis enabled Markovnikov hydroalkoxylation of alkenes. The precise control of the protons and electrons by three catalysts offers a new approach to valuable dialkyl ethers from readily available alcohols and alkenes without strong acids and external reductants/oxidants.
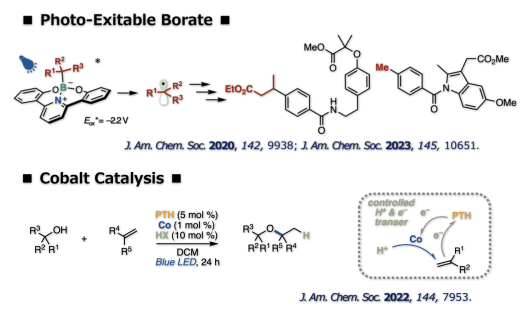

The direct visible-light excitation of organoboronate complex generated alkyl radicals without the need for an external photoredox catalyst. The photoexcitation of the borates is applicable to cross-couplings, thus enabling the introduction of various C(sp3) fragments to organic molecules. A visible light-driven triple photoredox/cobalt/Brønsted acid catalysis enabled Markovnikov hydroalkoxylation of alkenes. The precise control of the protons and electrons by three catalysts offers a new approach to valuable dialkyl ethers from readily available alcohols and alkenes without strong acids and external reductants/oxidants.


