Published to Nature Synthesis
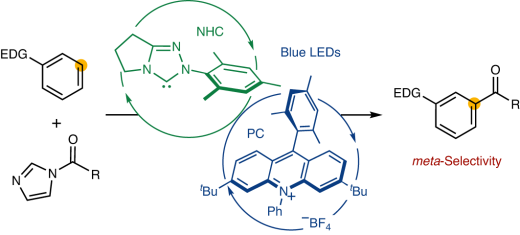
meta-Selective functionalisation of electron-rich arenes provides a non-traditional route to organic synthesis. In classical electrophilic aromatic substitution of electron-donating group-pendant arenes, functionalisation occurs according to ortho– and para-orientation. There have been numerous efforts to overcome this selectivity, and various synthetic methods have been developed, mainly based on transition metal catalysis. Here, we show a new N-heterocyclic carbene and organic photoredox cocatalysis for meta-selective acylation of electron-rich arenes. This approach proceeds without the directing groups or steric factors required in transition metal catalysis, resulting in precisely opposite regioselectivity from conventional approaches such as the Friedel–Crafts acylation. The catalytic system involves a sequence of single electron oxidation of an electron-rich arene followed by the radical–radical coupling between a ketyl radical and an arene radical cation. This protocol will lead to the expeditious synthesis of organic molecules that commonly require multiple steps and rare metals and promotes the construction of libraries of biologically active molecules.
DOI: 10.1038/s44160-023-00378-4