Just on ChemRxiv
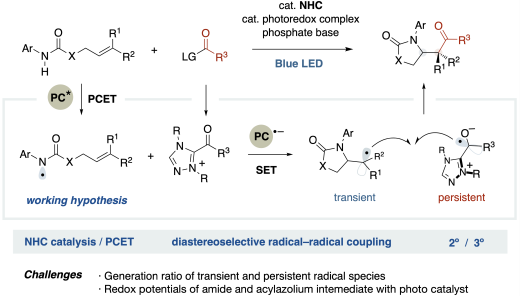
N-Heterocyclic carbene catalysis merging with photoredox PCET enabled amidoacylation of alkenes to construct sterically congested cyclic β-amido ketone skeleton bearing α-tertiary or quaternary carbon centers with high diastereoselectivity. The reaction involves generating amidyl radicals via oxidative PCET under photoredox catalysis, followed by rapid intramolecular cyclization to form the carbon-centered radical. The precise control of electron transfer in the two radical generation mechanisms is required to achieve this diastereoselective radical–radical coupling. The photocatalyst 3CzClIPN greatly facilitates this reaction based on its high oxidation potential and moderate reduction potential. The correlation between the redox potential of the photocatalyst and the yield provides an analogy for the reaction mechanism. Further transformation of the product afforded acyclic 3-amino butane-1,4-diol as a single diastereomer. This protocol allows the mild synthesis of structurally crowded bioactive molecules.
DOI: 10.26434/chemrxiv-2023-wbm3p