Published to Chem Catalysis
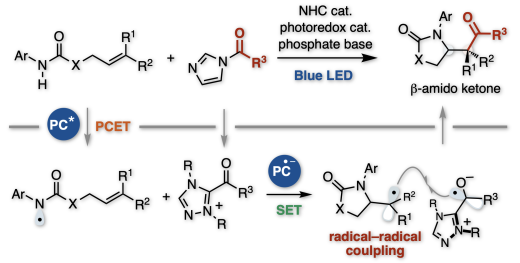
Merging N-heterocyclic carbene catalysis with photoredox proton-coupled electron transfer (PCET) has enabled the amidoacylation of alkenes for the construction of a sterically congested cyclic -amido ketone skeleton bearing -tertiary or -quaternary carbon centers with high diastereoselectivity. The reaction involves generating amidyl radicals via oxidative PCET under photoredox catalysis, followed by rapid intramolecular cyclization to form the carbon-centered radical. Precise control of electron transfer in the two- radical-generation mechanisms is required for achieving this diastereoselective radical-radical coupling. The photocatalyst 3CzClIPN greatly facilitates this reaction because of its high oxidation potential and moderate reduction potential. The correlation between the redox potential of the photocatalyst and the yield provides an analogy for the reaction mechanism. Further transformation of the product affords acyclic 3- aminobutane-1,4-diol as a single diastereomer. This protocol allows the mild synthesis of structurally crowded bioactive molecules.
DOI: 10.1016/j.checat.2023.100736