Published to Organic Letters
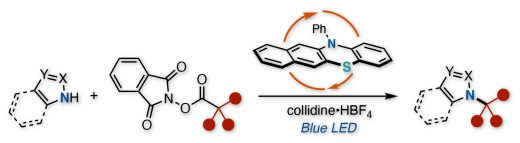
Our new paper by a collaborative work with Takeda Pharm has been published to Org.Lett. This manuscript demonstrates an organophotoredox-catalyzed decarboxylative cross-coupling between azole nucleophiles and aliphatic carboxylic acid-derived redox active esters. This protocol efficiently installs various tertiary or secondary alkyl fragments onto the nitrogen atom of azole nucleophiles under mild and transition-metal-free conditions. The pyridinium additive successfully inhibits the formation of elimination byproducts from the carbocation intermediate. This reaction is applicable to the synthesis of a protein degrader-like molecule containing an azole and a thalidomide.
DOI: 10.1021/acs.orglett.1c01745