Published to Journal of the American Chemical Society
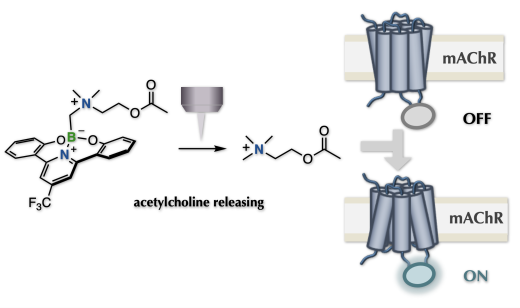
Photo-caged methodologies have been indispensable for elucidating the functional mechanisms of pharmacologically active molecules at the cellular level. A photo-triggered removable unit enables control of the photo-induced expression of pharmacologically active molecular function, resulting in a rapid increase in the concentration of the bioactive compound near the target cell. However, caging the target bioactive compound generally requires specific heteroatom-based functional groups, limiting the types of molecular structures that can be caged. We have developed an unprecedented methodology for caging/uncaging on carbon atoms using a unit with a photo-cleavable carbon–boron bond. The caging/uncaging process requires installation of the CH2–B group on the nitrogen atom that formally assembles an N-methyl group protected with a photoremovable unit. N-methylation proceeds by photoirradiation via carbon-centered radical generation. Using this radical caging strategy to cage previously uncageable bioactive molecules, we have photocaged molecules with no general labelling sites, including acetylcholine, an endogenous neurotransmitter. Caged acetylcholine provides an unconventional tool for optopharmacology to clarify neuronal mechanisms on the basis of photo-regulating acetylcholine localization. We demonstrated the utility of this probe by monitoring uncaging in HEK cells expressing a biosensor to detect ACh on the cell surface, as well as Ca2+ imaging in Drosophila brain cells (ex vivo).
DOI: 10.1021/jacs.3c00801