Published to Chem
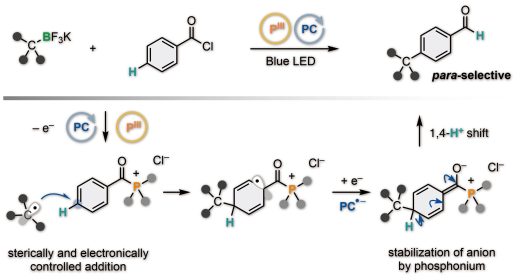
Site-selective C(sp2)–H alkylation of arenes has served ubiquitous fragments found in pharmaceutical drugs and agrochemicals. While ortho– and meta-selective alkylation reactions have been achieved well, para-selective examples are scarce. Currently available methods have been designed on unique behaviors and characteristics of metals to achieve high para selectivity. Herein, we demonstrate para-selective C(sp2)–H alkylation of aroyl chlorides with alkyltrifluoroborates and trialkylphosphine through an organic photoredox-catalyzed radical tele-substitution. The reaction proceeds through para-selective addition of alkyl radicals to in situ generated aroyl trialkylphosphonium intermediates followed by hydrogen shift. Notably, the para-alkylation occurred associated with conversion of carbonyl trialkylphosphonium group to formyl group, producing para-alkylated benzaldehyde derivatives. This unprecedented metal-free site-selective C(sp2)–C(sp3) bond formation allows for streamlined synthesis of Siponimod and SR-31747.
DOI: 10.1016/j.chempr.2025.102446