Published to Angewandte Chemie
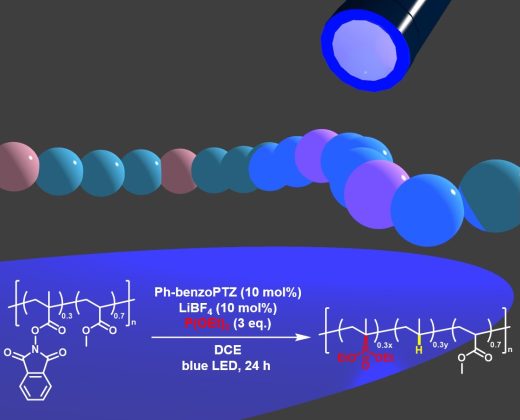
Photoredox-catalyzed postfunctionalization of poly(meth)acrylate-based copolymers bearing phthalimide moieties can produce novel polymer architectures that are inaccessible using polymerization approaches; however, this method is restricted to radical reactions. To expand the scope of reactions applicable to photoredox-catalyzed postfunctionalization, this study focused on the radical–polar crossover (RPC) process, in which a carbocation equivalent is generated to react with nucleophiles. The organophotoredox-catalyzed reaction of a random copolymer of N-(methacryloxy)phthalimide and methyl acrylate using trialkyl phosphite nucleophiles afforded the corresponding phosphonate-containing polymers. Although simultaneous hydrogenation was inevitable, a novel polymer comprising dialkyl isopropenylphosphonate, propylene, and methyl acrylate units was obtained, which is of interest because phosphonate-containing polymers are potentially applicable as thermoresponsive materials, flame-retardant materials, and additives in Li-ion batteries. A series of copolymers with different components were successfully applied to the postfunctionalization method. The organophotoredox catalysis-based postfunctionalization strategy developed in this study provides versatile functional polymers in a simple, green, and sustainable manner.
DOI: 10.1002/anie.202507572