Tetrahedron Letters 誌に掲載
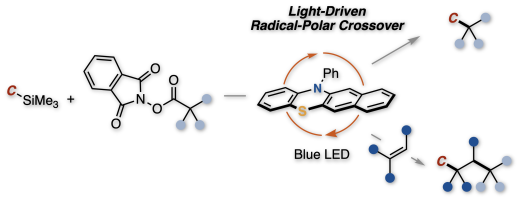
In this communication, we report a visible light-mediated organophotoredox catalysis enabling the cross- coupling between aliphatic carboxylic acid-derived redox active esters and organosilanes. In this proto- col, a carbocation equivalent generated from the redox active ester by radical-polar crossover process could react with an organosilane reagent. This protocol allows to forge C(sp3)–C(sp3), C(sp3)–C(sp2) and C(sp3)–C(sp) bonds under transition-metal free and mild reaction conditions. The privileged feature of radical-mediated process was demonstrated as radical-relay type dicarbofunctionalization of styrene derivatives using organosilanes and aliphatic redox active esters.
DOI: 10.1016/j.tetlet.2022.154231