Nature Communications 誌に掲載
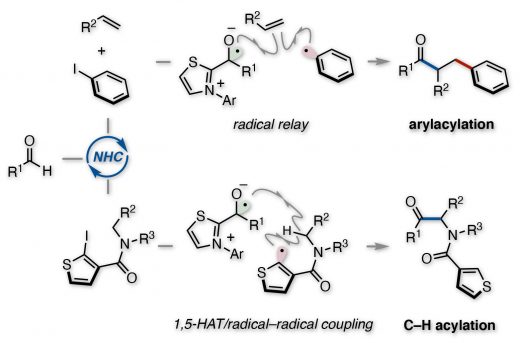
The paper on aryl radical-mediated N-heterocyclic carbene catalysis is now out in Nature Communications. There have been significant advancements in radical reactions using organocatalysts in modern organic synthesis. Recently, NHC-catalyzed radical reactions initiated by single electron transfer processes have been actively studied. However, the reported examples have been limited to catalysis mediated by alkyl radicals. In this article, the NHC organocatalysis mediated by aryl radicals has been achieved. The enolate form of the Breslow intermediate derived from an aldehyde and thiazolium-type NHC in the presence of a base undergoes single electron transfer to an aryl iodide, providing an aryl radical. The catalytically generated aryl radical could be exploited as an arylating reagent for radical relay-type arylacylation of styrenes and as a hydrogen atom abstraction reagent for α-amino C(sp3)–H acylation of secondary amides.
DOI: 10.1038/s41467-021-24144-2